Noncanonical inflammasome signaling elicits gasdermin D-dependent neutrophil extracellular traps
The Inflammasome Lab have discovered a new pathway for host defence against cytosolic Gram-negative bacteria, revealing unexpected links between pyroptosis and NETosis.
Chen KW1, Monteleone M1, Boucher D1, Sollberger G, Ramnath D, Condon ND, von Pein JB, Broz P, Sweet MJ, Schroder K. (2018).
Noncanonical inflammasome signaling elicits gasdermin D-dependent neutrophil extracellular traps. Science Immunology Aug 24;3(26). pii: eaar6676. Pubmed
1Equal contribution
Editor's summary
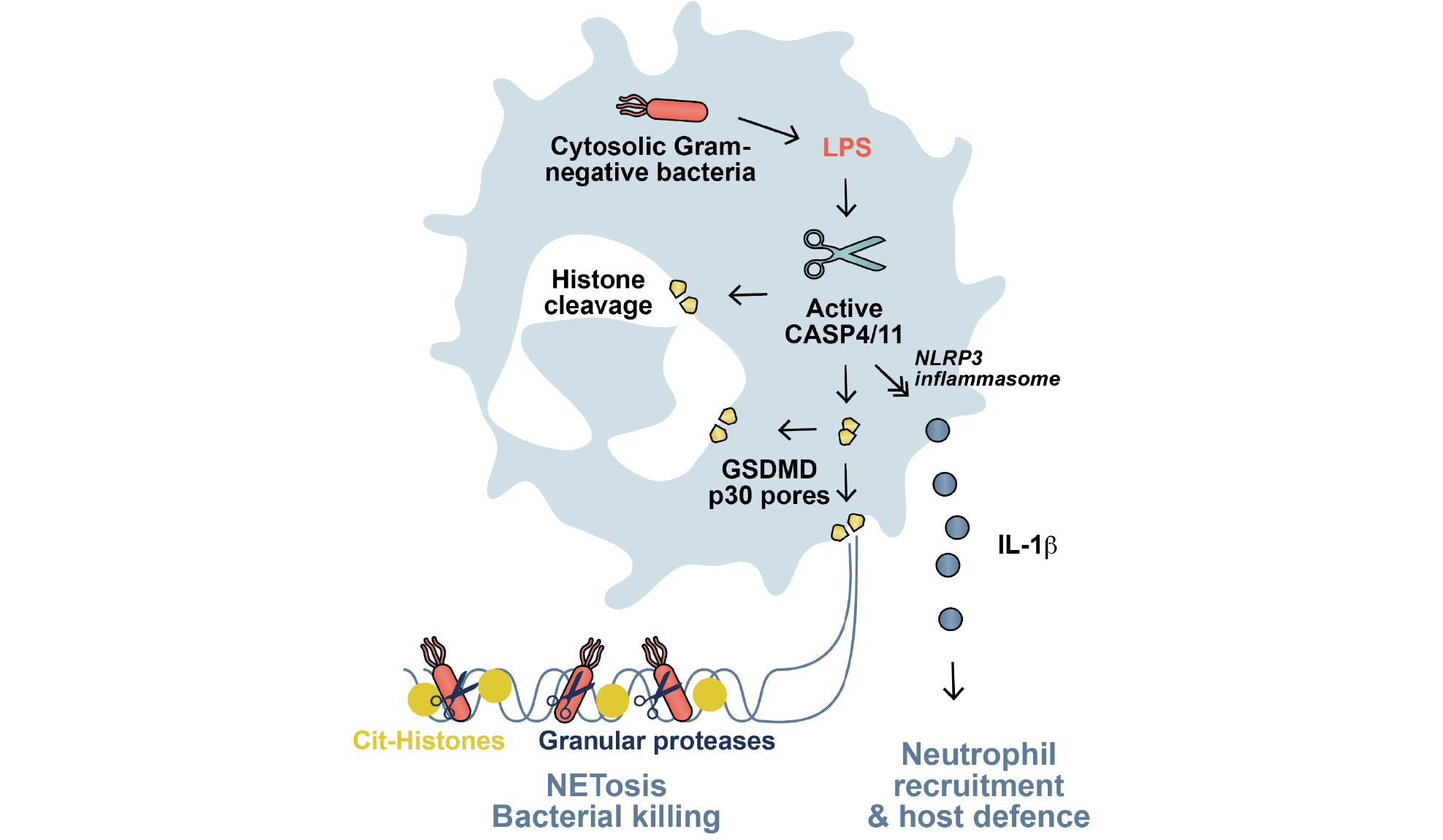
Gasdermin D (GSDMD), a pore-forming protein, has emerged a key downstream effector in pyroptosis, a form of cell death induced by intracellular lipopolysaccharide (LPS). Here, by examining the role of GSDMD in the neutrophil response to LPS and cytosolic Gram-negative bacteria, Chen et al. have uncovered an important role for GSDMD in the generation of neutrophil extracellular traps (NETs). NETs are composed of chromatin and antimicrobial proteins and are cast by dying neutrophils in a process termed NETosis. The authors report that GSDMD is directly cleaved by caspase-11 and that intracellular LPS–induced NETosis is dependent on both caspase-11 and GSDMD. In the same issue, Sollberger et al. also report a role for GSDMD in NETosis.
Abstract
Neutrophil extrusion of neutrophil extracellular traps (NETs) and concomitant cell death (NETosis) provides host defense against extracellular pathogens, whereas macrophage death by pyroptosis enables defense against intracellular pathogens. We report the unexpected discovery that gasdermin D (GSDMD) connects these cell death modalities. We show that neutrophil exposure to cytosolic lipopolysaccharide or cytosolic Gram-negative bacteria (Salmonella ΔsifA and Citrobacter rodentium) activates noncanonical (caspase-4/11) inflammasome signaling and triggers GSDMD-dependent neutrophil death. GSDMD-dependent death induces neutrophils to extrude antimicrobial NETs. Caspase-11 and GSDMD are required for neutrophil plasma membrane rupture during the final stage of NET extrusion. Unexpectedly, caspase-11 and GSDMD are also required for early features of NETosis, including nuclear delobulation and DNA expansion; this is mediated by the coordinate actions of caspase-11 and GSDMD in mediating nuclear membrane permeabilization and histone degradation. In vivo application of deoxyribonuclease I to dissolve NETs during murine Salmonella ΔsifA challenge increases bacterial burden in wild-type but not in Casp11−/− and Gsdmd −/− mice. Our studies reveal that neutrophils use an inflammasome- and GSDMD-dependent mechanism to activate NETosis as a defense response against cytosolic bacteria.
See Science Immunology for a complete list of citations, reviews and media attention this publication has received. Read the news articles here
Highlight:
New pathway in body’s immune response uncovered. UQ News
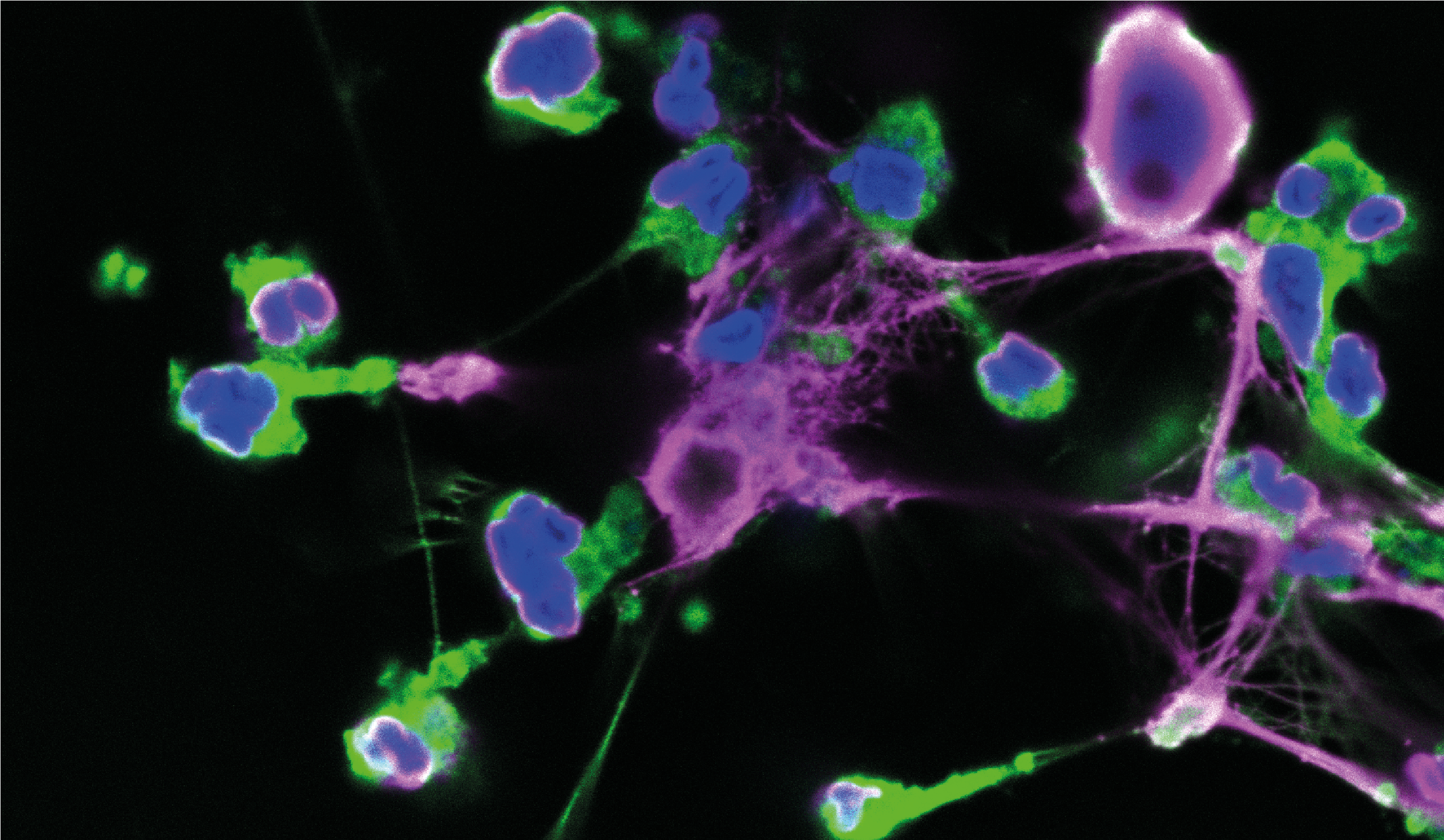
Image: Human neutrophils casting neutrophil extracellular traps upon infection with C. rodentium, imaged by confocal microscopy, with staining for myeloperoxidase (green), extracellular chromatin (red) and total DNA (DAPI, blue). Image credit: Dr Gabriel Sollberger, Max Planck Institute for Infection Biology, Germany.
Copyright © 2017 - The University of Queensland