The Salmonella pathogenicity island‐2 subverts human NLRP3 and NLRC4 inflammasome responses
Here the Inflammasome Lab reveal that Salmonella evades immune response by subverting the NLRP3 and NLRC4 inflammasome in macrophages.
Damien Bierschenk, Mercedes Monteleone, Fiona Moghaddas, Seth L. Masters, Dave Boucher, Kate Schroder. (2018).
The Salmonella pathogenicity island‐2 subverts human NLRP3 and NLRC4 inflammasome responses. Journal of Leukocyte Biology 2019;105:401–410. Pubmed
Abstract
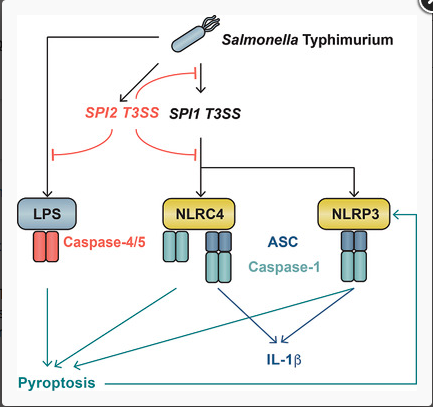
Inflammasomes are signaling hubs that activate inflammatory caspases to drive cytokine maturation and cell lysis. Inflammasome activation by Salmonella Typhimurium infection or Salmonella‐derived molecules is extensively studied in murine myeloid cells. Salmonella‐induced inflammasome signaling in human innate immune cells, is however, poorly characterized. Here, we show that Salmonella mutation to inactivate the Salmonella pathogenicity island‐2 type III secretion system (SPI2 T3SS) potentiates S. Typhimurium‐induced inflammasome responses from primary human macrophages, resulting in strong IL‐1β production and macrophage death. Inactivation of the SPI1 T3SS diminished human macrophage responses to WT and ΔSPI2 Salmonella. Salmonella ΔSPI2 elicited a mixed inflammasome response from human myeloid cells, in which NLR family CARD‐domain containing protein 4 (NLRC4) and NLR family PYRIN‐domain containing protein 3 (NLRP3) perform somewhat redundant functions in generating IL‐1β and inducing pyroptosis. Our data suggest that Salmonella employs the SPI2 T3SS to subvert SPI1‐induced NLRP3 and NLRC4 inflammasome responses in human primary macrophages, in a species‐specific immune evasion mechanism.
Image: The Gram‐negative bacterium Salmonella Typhimurium suppresses human macrophage inflammasome responses via the Salmonella Pathogenicity Island 2.
Copyright © 2017 - The University of Queensland